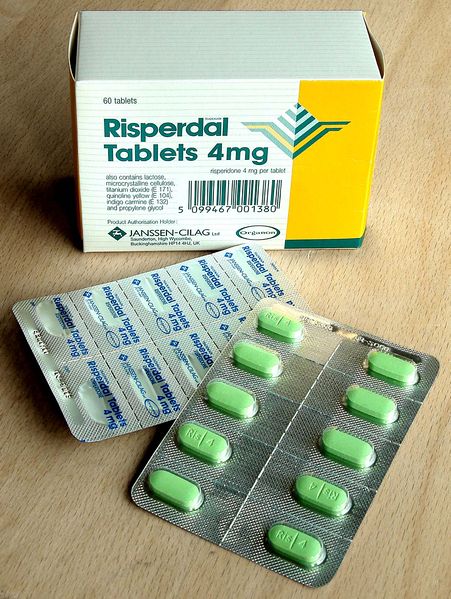
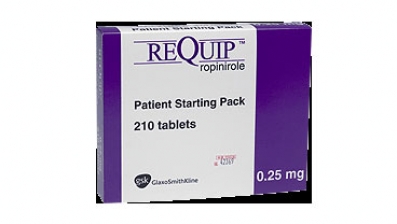
The US Food and Drug Administration has issued an alert about medication error reports in which patients were mistakenly given the antipsychotic risperidone (Risperdal, Johnson & Johnson) instead of the dopamine agonist ropinirole (Requip, GlaxoSmithKline) and vice versa.
The FDA evaluated 226 wrong-drug medication errors relating to confusion between the 2 drugs from the Adverse Event Reporting System database and the Institute for Safe Medication Practices. Several cases resulted in adverse events (n = 16), including 5 cases that required patients to be hospitalized.
The adverse events included confusion, lethargy, ataxia, hallucinations, tiredness, dizziness, tingling, numbness, and altered mental status. The FDA reports that in 1 case, reported outside the United States, a patient was given risperidone instead of ropinirole for 1 month before the medication error was noticed.
Risperidone was restarted without benefit of titration. One month after the drug was restarted the patient died. However, the FDA reports it is not clear "what role, if any, the error had in the death of this patient."
The FDA determined that the factors contributing to the confusion between the 2 products include the following:
- Similarities of both brand (proprietary) and generic (established) names;
- Similarities of the container labels and carton packaging;
- Illegible handwriting on prescriptions; and
- Overlapping product characteristics, such as the drug strengths, dosage forms, and dosing intervals.
Further, the FDA is requesting that the manufacturers of the brand-name medications and their generic counterparts take the following measures to reduce the potential for confusion between the 2 products:
- Use of "tall man" lettering on container labels and carton packaging to present generic names as risperidone and rOPINIRole, which may improve the ability of healthcare professionals to distinguish between the 2 drug names.
- Change individual labels and carton packaging to provide better visual differentiation between the generic products for risperidone and ropinirole in order to reduce the potential for confusion. Currently, the label and packaging features (ie, similar font size and type, layout, and color) for generic ropinirole and risperidone products make the bottles look similar.
The FDA notes that the incidence of confusion between the 2 products increased considerably after 2006 with the introduction of the generic products, which feature the established generic names on container labels. Generic risperidone was approved in 2006 and generic ropinirole was approved in 2008. Nevertheless, the FDA says it cannot rule out the possibility that the brand names may also have contributed to the confusion between the 2 drugs.
Medical errors related to confusion between risperidone and ropinirole should be reported to MedWatch by telephone at 1-800-332-1088, by fax at 1-800-332-1078, online at https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm, or by mail to MedWatch, 5600 Fishers Lane, Rockville, Maryland 20857.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 