很多人都會跟你說葉黃素酯吸收比較差,所以我們來研究研究
Esterification Does Not Impair Lutein Bioavailability in Humans
The Journal of Nutrition, Volume 132, Issue 12, December 2002, Pages 3668–3673, https://doi.org/10.1093/jn/132.12.3668
ABSTRACT
Age-related macular degeneration (ARMD) is inversely associated with the accumulation of lutein + zeaxanthin in the macula, but higher lutein intakes are inconsistently related to reduced risk of ARMD in epidemiologic studies. Resolution of efficacy awaits clinical trials designed with knowledge of lutein supplement pharmacokinetics. Lutein bioavailability was determined for lutein diester and unesterified lutein formulations as they might be incorporated into dietary supplements. Healthy subjects (n = 18) consumed a single dose of each formulation (either 0.5 or 0.67 μmol lutein/kg body, 10 and 8 subjects, respectively) in random order, and the appearance of free lutein + zeaxanthin was measured in serum from 0 to 408 h. Areas under the serum concentration × time curves (AUC), as a measure of bioavailability, were independent of gender, body mass index and lutein dose. The lutein diester formulation was 61.6% more bioavailable than the unesterified lutein formulation with higher mean AUC, maximum serum concentration and ascending slope (P < 0.05). The AUC was greater in 14 of 18 subjects when they consumed the lutein diester formulation. Comparison with data from previous studies suggested that dissolution was a greater limitation to bioavailability than lutein ester hydrolysis because an oil-solubilized unesterified lutein preparation, given at 0.5 μmol/kg body, resulted in greater mean peak concentrations and AUC compared with either the unesterified or lutein diester formulations used in our study. In conclusion, the lutein diester formulation poses no impediment to lutein bioavailability at the doses tested, but formulation dissolution is an important factor in lutein bioavailability and should be evaluated before a supplement and dose are selected for use in clinical trials.
Age-related macular degeneration (ARMD)3 is the major cause of irreversible blindness in older men and women. The prevalence of ARMD in the 55- to 64-y-old age group from the United States, Europe and Australia is 0.2% but rises to 13% for those >85 y old (1). Maculopathy is more prevalent in patients with Type 1 and 2 diabetes at 42 and 53%, respectively (2). The risk of ARMD is inversely proportional to macular pigment density (3,4), which is directly proportional to the selective accumulation of the carotenoids, lutein and zeaxanthin in human and primate retinas (5,6). The proposed protective effect of lutein and zeaxanthin accumulation is their ability to absorb light in the blue wavelengths that impinge directly on the fovea of the retina (7) and the special ability of carotenoids to quench singlet oxygen and other reactive oxygen species, thus protecting the macular cells during a lifetime of oxidative stress (8). Diet intakes and plasma concentrations of lutein + zeaxanthin ≥5.8 mg/d and ≥0.67 μmol/L, respectively, were associated with the lowest risk for ARMD (9,10). Yet, other studies have not found such an association (11), or an inverse association was found only after adjusting for age, gender, alcohol use, hypertension, smoking and body mass index (12). One of the problems in demonstrating an association between lutein + zeaxanthin intakes and the prevalence or incidence of ARMD is that the bioavailability of lutein from different food sources is extremely variable, e.g., it is high from egg yolk (13) and very low from green leafy vegetables (14). Furthermore, there is great variability among individuals in the macular accumulation of lutein from foods (15), which is complicated by the observation that macular pigment densities remain high long after supplementation and plasma levels have decreased (16). Before contemplating clinical trials for determining whether lutein or zeaxanthin may reduce the risk for ARMD, a thorough understanding of lutein supplement bioavailability and its variance in target populations is necessary. Kostic et al. (17) evaluated the pharmacokinetics of a single dose of free lutein and a combination of free lutein and β-carotene in humans, but they dissolved the carotenoids in a larger volume of oil than would be practical for intervention trials carried out over a number of years. The bioavailability of esterified lutein, found at low levels in many fruits and vegetables (18,19), and the predominant form found in marigold petals (20), has not been systematically evaluated. The objective of this study was to compare the bioavailability of esterified and unesterified lutein in formulations as they might be included in dietary supplements, in human subjects.
SUBJECTS AND METHODS
The study used a single-dose, randomized, crossover design. Subjects consumed a self-selected diet low in carotenoids for 1 wk, then came to the Nutrition and Metabolism Research Laboratory (NMRL) in a fasting state on d 1 of each dosing. After a baseline blood sample, subjects consumed the assigned supplement and immediately thereafter ate a provided breakfast. Blood was sampled at 2, 4, 6, 8, 10, 12, 16, 24, 34, 72, 120, 240 and 408 h after baseline. Lunch was 4 h and dinner 8 h after supplementation on d 1. Standard meals were also supplied on d 2. One week after the last blood draw, subjects repeated the procedure with the other supplement. Subjects were required to consume a low carotenoid diet throughout the study.
Subject selection.
Subjects were recruited from staff and students in the university community via posted announcements. Subjects were men or women between the ages of 22 and 35 y, nonsmokers, within ± 15% of their ideal body weight (21), had no chronic diseases or gastrointestinal disturbances, and were taking no prescription or over-the-counter drugs during the study, or vitamin or mineral supplements containing carotenoids. The study was approved by the Institutional Review Board for Human Subjects Research of the University of Illinois at Chicago.
Twenty subjects started the study, but one became ill with nausea and headache during d 1 of the study and one subject was found to be pregnant between doses, leaving 18 subjects who completed both doses of the study: 8 women and 10 men were included in all data analyses. Sample size calculations were based upon data presented by Kostic et al. (17), who gave 0.5 μmol lutein/kg body (Kemin Industries, Des Moines, IA) to 8 subjects (men and women) and calculated the 0–440 h area under the serum lutein concentration × time curve (AUC) to be 59.6 ± 25.5 μmol/(L · h). Eighteen subjects were estimated to be necessary to detect a 30% difference between the mean AUC of the two lutein formulations (1-β = 80%, α = 0.05, two-tailed test).
Lutein formulations.
The unesterified lutein formulation, 20 g/100 g lutein derived from marigold flowers, was a crystalline suspension in safflower oil containing vitamin E, rosemary and citric acid as preservatives (Kemin). Carotenoid composition was reported to be 70% all-trans lutein and 3–7% all-trans zeaxanthin. The lutein ester formulation was reported to be 36.7 g/100 g lutein in the esterified form, derived as a powder from marigold flowers (Cognis, Nutrition and Health Group, LaGrange, IL, formerly Henkel). The carotenoid composition was reported to be 93.4% all-trans lutein, 0.29% cis lutein, 4.0% all-trans zeaxanthin and small amounts of other carotenoids. The ester composition was reported to be 56% lutein dipalmitate, 36% lutein dimyristate and 8% monomyristate. Doses were calculated on the basis of the all-trans lutein content of each formulation, so that the same amount of all-trans lutein was delivered in each formulation. Ten subjects received 0.5 μmol lutein/kg body from both formulations (mean = 20.7 mg) and 8 subjects received 0.67 μmol/kg body (mean = 24.5 mg). Each formulation was packed into a gelatin capsule to deliver the appropriate dose to each subject. The order of treatment was randomly assigned.
Diets.
All subjects consumed prepared diets for 48 h postdosing, which contained negligible quantities of all carotenoids, 30% of energy as fat at each meal, with 20, 30 and 50% of energy contributed by breakfast, lunch and dinner, respectively. This approximates the usual U.S. adult population meal pattern. Breakfasts consisted of a bagel with cream cheese, low fat yogurt, apple juice and contained 11.6–23 g total fat. The macronutrient and antioxidant compositions of selected energy levels (Nutritionist IV, N-Square Computing, Salem, OR) are presented in Table 1. Diets ranging from 7.524 to 15.884 MJ were calculated and assigned to subjects on the basis of their estimated total energy expenditure (TEE). Subjects were given a list of allowable fruits and vegetables (low in carotenoids) as well as a list of foods to avoid (containing moderate-to-large amounts of lutein and other carotenoids). They were instructed to adhere to the diet for the week before the study and until the last blood draw after the second dose. Diet records were collected on selected days throughout the study to monitor adherence to the diet recommendations.
Macronutrient and antioxidant concentrations of the controlled diet for selected energy levels consumed by men and women for 2 d after single doses of lutein or lutein diester formulations1
| Dietary energy | |||||
|---|---|---|---|---|---|
| Energy level,2MJ/d | 8.360 | 11.704 | 14.226 | ||
| Fat, g/d | 57 | 86 | 110 | ||
| Fat, en% | 29 | 29 | 29 | ||
| Protein, g/d | 73 | 101 | 124 | ||
| Protein, en% | 16 | 15 | 14 | ||
| Carbohydrate, g/d | 257 | 364 | 490 | ||
| Carbohydrate, en% | 56 | 55 | 57 | ||
| Dietary fiber, g/d | 0.1 | 0.1 | 0.1 | ||
| Vitamin A,3RE/d | 350 | 453 | 511 | ||
| Vitamin C, mg/d | 125 | 128 | 132 | ||
| Vitamin E, mg/d | 10.7 | 11.8 | 17.8 | ||
Diets ranged from 7.524 to 15.884 MJ/d and all nutrients were calculated using Nutritionist IV.
4.18 MJ = 1000 kcal.
From preformed retinol. RE, retinal equivalents.
Protocol.
The subjects were divided into two groups and 9 were evaluated at a time. They came to the NMRL in a fasting state on the morning of d 1. A butterfly catheter was inserted into an arm vein and 5 mL of blood was drawn for a baseline value; then esterified or unesterified lutein, weighed into gelatin capsules (according to the randomization schedule), was consumed with 250 mL of water followed by breakfast. Then, 3-mL blood samples were drawn through the catheter kept patent with heparin 4, (lunch) 6, 8, (dinner) 10, 12 and 16 h later. Subjects were then sent home (catheter removed) with instructions to not consume any food or beverage other than water. They returned to the NMRL the next morning and blood was sampled 24 h (d 2) after the baseline blood draw, and again at 34 h on d 2. The same low carotenoid diet was provided to each subject during d 2. They returned for fasting morning blood draws 72, 120, 240 and 408 h after baseline.
Carotenoid analysis.
Blood was allowed to clot and serum separated and frozen at −80°C until analyzed for free lutein. All serum samples from an individual were analyzed together to minimize between-analysis variability. Samples were analyzed in duplicate after hexane extraction by HPLC, using the method of Stacewicz-Sapuntzakis et al. (22). Lutein, zeaxanthin and β-cryptoxanthin standards were obtained from Extrasynthese (Genay, France) and α-carotene, β-carotene and lycopene standards were obtained from Sigma Chemical (St. Louis, MO). The detection limit for the assay was 3.5 nmol/L for lutein + zeaxanthin and the between-assay CV for 14 assays of control serum was 2.4%. Our laboratory participates in the National Institute of Standards Technology Micronutrient Quality Assurance Program and our reported values are always within 1 SD of the mean of all participating laboratories (23). Lutein was not separated from zeaxanthin in this study and the lutein + zeaxanthin measurements will be referred to as lutein hereafter.
Analysis of results.
Subjects were used as their own controls for all analyses. Areas under the serum concentration × time curves (AUC) were calculated using baseline uncorrected and corrected values for each individual according to the following procedures: 1) Without Correction for Baseline: the AUC was calculated from 0 to 408 h using the measured lutein concentrations for each time point. The baseline lutein concentration was not subtracted from any value. This procedure produced lower CV and normal distributions. 2) With Correction for Baseline: a regression line was constructed using the serum lutein values at baseline and 408 h, which took into account the decrease in fasting serum lutein concentrations due to the consumption of a low carotenoid diet during the study period. The regression-calculated baseline lutein concentrations for each measured time point were subtracted from the corresponding measured serum lutein concentrations. AUC were normally distributed only after log transformation. Baseline-corrected values were used for comparisons to published data and proportional differences could be calculated. AUC for both procedures were calculated by the trapezoidal rule.
All data were entered and manipulated using Excel spreadsheets (Microsoft, Seattle, WA). Baseline-corrected AUC, peak height, time to peak and slopes for each dose required log transformation. The probability of true differences was estimated by two-tailed paired t test. The nonparametric Mann-Whitney test was applied to untransformed data to assess differences in median AUC. Correlations by gender, body mass index (BMI) and baseline serum cholesterol and lutein concentrations were calculated on log-transformed data. Inferential statistical tests were performed using Graph Pad InStat (GraphPad Software, San Diego, CA) or Microsoft Excel and a level of 0.05 was set for significance of differences and associations.
RESULTS
Study population.
Subjects represented a distribution of ethnicities and gender (Table 2). They took no medication or vitamin supplements during the study and consumed a low carotenoid diet during the study period. BMI of men and women were similar.
Anthropometric characteristics of study subjects1
| Men | Women | |
|---|---|---|
| n | 10 | 8 |
| Age, y | 25.2 ± 4.4 | 27.6 ± 3.9 |
| Body weight, kg | 74.0 ± 9.2 | 63.0 ± 5.4 |
| Height, m | 1.73 ± 0.93 | 1.64 ± 0.74 |
| Body mass index, kg/m2 | 24.6 ± 2.6 | 23.5 ± 2.3 |
| Ethnicity, n | ||
| African-American | 1 | 1 |
| Asian-American | 2 | 2 |
| Euro-American | 7 | 5 |
| Hispanic-American | 0 | 0 |
Values are mean ± SD or n.
AUC without baseline adjustment.
Because AUC, calculated without baseline correction, were normally distributed and had smaller CV, comparisons as a function of gender, BMI, dose and formulation using baseline uncorrected data, are presented in Table 3. The mean AUC was greater for the lutein diester formulation, indicating greater bioavailability than the unesterified lutein formulation. Gender, BMI or dose did not explain the difference in apparent bioavailability between the two formulations. Furthermore, the overall bioavailability of either formulation was not affected by gender or BMI and there was no dose response. However the differences in dose were small and the study did not have sufficient power to determine that differences did not exist between the various subgroups, i.e., men vs. women, low BMI vs. high BMI, lower vs. higher dose.
Areas under the serum lutein concentration × time curves without correction for baseline as a function of gender, body mass index (BMI), dose and lutein formulation in response to single doses of lutein and lutein diester1
| Parameter | n | Lutein Diester | Lutein | P-value |
|---|---|---|---|---|
| μmol/(L · h) | ||||
| Total group | 18 | 159 ± 482 | 138 ± 49 | 0.01 |
| Men3 | 10 | 166 ± 55 | 145 ± 60 | 0.04 |
| Women | 8 | 149 ± 38 | 128 ± 31 | 0.13 |
| BMI,3 25.1–28.01 kg/m2 | 9 | 78 ± 51 | 144 ± 53 | 0.01 |
| BMI, 21.0–23.3 kg/m2 | 9 | 139 ± 38 | 131 ± 46 | 0.37 |
| Higher dose3 (0.67 mmol/kg body) | 8 | 155 ± 40 | 131 ± 33 | 0.17 |
| Lower dose (0.5 mmol/kg body) | 10 | 161 ± 53 | 142 ± 58 | 0.03 |
Values are means ± SD, n = 18.
Comparisons by paired t test without Bonferroni correction.
All comparisons for men vs. women, lower vs. higher BMI and lower vs. higher dose within each formulation by unpaired t test without Bonferroni correction were not significant.
Kinetic comparison of lutein and lutein diester formulations with baseline adjustment.
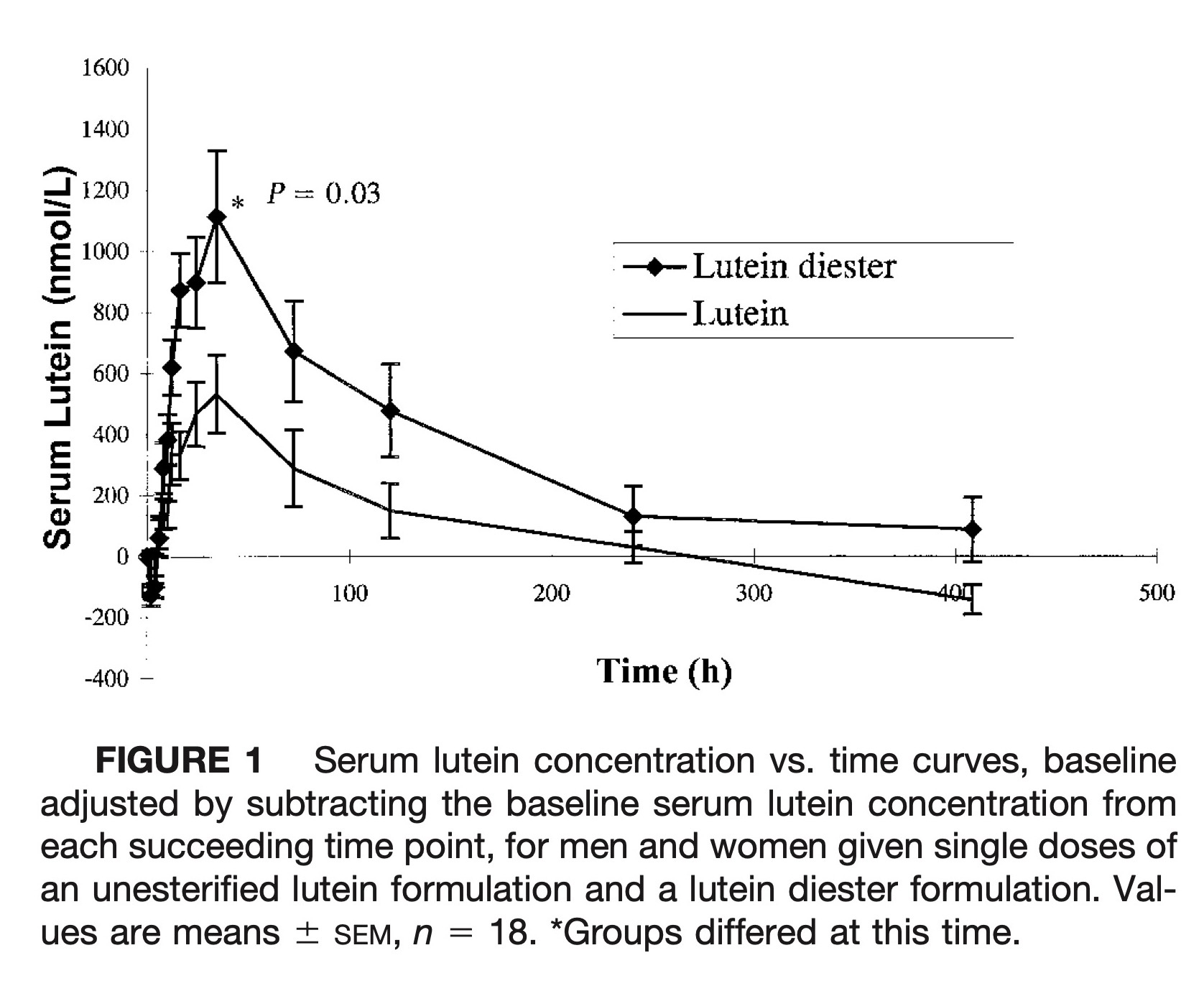
The mean serum lutein response curves for the lutein diester and unesterified lutein formulations, in which the baseline serum lutein concentration was subtracted from each value is presented in Figure 1. The mean serum lutein concentration crossed the baseline for the free lutein formulation at 270 h, reflecting the effect of the low carotenoid diet on serum lutein concentrations. Mean peak concentration for both lutein formulations was achieved at ∼32 h with considerable variation among subjects. There was no discernible peak at 4–6 h, which is often observed in β-carotene single-dose studies (17). The serum concentration time curve characteristics adjusted for baseline lutein concentrations, where appropriate, are presented in Table 4. Mean baseline serum concentrations of lutein and total cholesterol did not differ when subjects were given each formulation. Lutein concentrations were not cholesterol-adjusted because there was no correlation between lutein and total cholesterol concentrations at baseline. Free lutein from the lutein diester formulation appeared in the peripheral circulation more quickly, attained a higher peak concentration and the mean AUC was 61.6% greater (the median AUC was 38% greater) than after treatment with the unesterified lutein formulation (P = 0.03).
Serum lutein concentration vs. time curves, baseline adjusted by subtracting the baseline serum lutein concentration from each succeeding time point, for men and women given single doses of an unesterified lutein formulation and a lutein diester formulation. Values are means ± SEM, n = 18. *Groups differed at this time.
Areas under the serum lutein concentration × time curves (AUC) with correction for baseline in subjects given single doses of lutein and lutein diester,1, 2
| Serum parameter | Lutein diester | Lutein | P-value3 | Lutein in oil |
|---|---|---|---|---|
| Mean baseline total cholesterol, mmol/L | 4.56 ± 0.73 | 4.31 ± 0.61 | NS4 | — |
| Mean baseline lutein, nmol/L | 315 ± 116 | 314 ± 121 | NS | 300 ± 110 |
| Mean lutein maximum concentration,5nmol/L | 200 ± 120 | 110 ± 100 | 0.03 | 870 ± 133 |
| Mean time to peak, h | 33.3 (8–120)6 | 31.1 (12–73) | NS | 16 (12–32) |
| Mean 408 h lutein, nmol/L | 330 ± 120 | 290 ± 110 | 0.04 | — |
| Mean ascending slope, nmol/(L · h) | 10 ± 6 | 4 ± 4 | 0.003 | — |
| Mean descending slope, nmol/(L · h) | −0.7 ± 0.8 | −0.4 ± 0.7 | NS | — |
| Mean AUC,5nmol/(L · h) | 37.0 ± 35.8 | 22.9 ± 19.0 | 0.03 | 59.6 ± 25.5 |
| Median AUC,5nmol/(L · h) | 24.4 | 17.7 | 0.097 | — |
| 17.1–33.28 | 7.2–35.3 |
Values are means± SD.
Data from Kostic et al. (17). Eight young adults consumed a single dose of 0.5 μmol/kg body of lutein in true oil solution. Blood was sampled 13 times over 840 h. Serum free lutein+ zeaxanthin was measured. AUC was calculated using baseline-corrected serum lutein+ zeaxanthin concentrations. The only differences in design between our study and that of Kostic et al., were that 8/18 of our subjects received a single dose of 0.67 μmol/kg body and we discontinued blood sampling at 408 h because data from Kostic et al. indicated that their subjects had reached baseline values by this time.
Comparisons by paired t test on log-transformed data.
NS, P ≥ 0.05.
Concentrations are baseline corrected.
Mean (range).
Paired comparisons using the Mann-Whitney test.
AUC interquartile range: 25th–75th percentile.
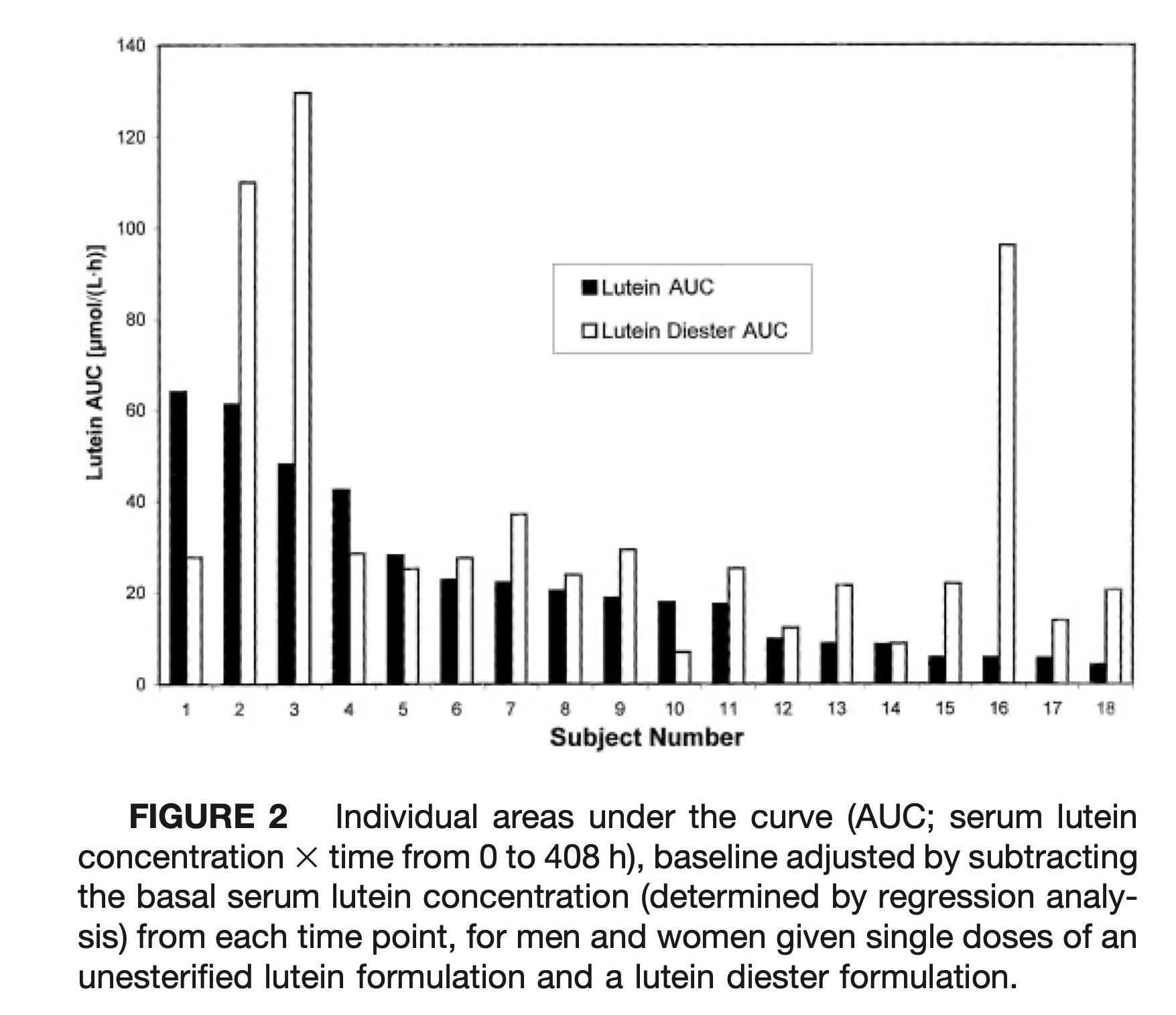
The paired baseline adjusted AUC comparisons of the two lutein formulations for each individual are presented in Figure 2. Apparent absorption of lutein from the lutein diester formulation was greater in 14 of the 18 subjects (78%). With a few outstanding exceptions, subjects generally had either good or poor bioavailability from both formulations. The correlation between AUC for each subject was r = 0.42 but was not significant (P = 0.08).
Individual areas under the curve (AUC; serum lutein concentration × time from 0 to 408 h), baseline adjusted by subtracting the basal serum lutein concentration (determined by regression analysis) from each time point, for men and women given single doses of an unesterified lutein formulation and a lutein diester formulation.
DISCUSSION
Surprisingly, the lutein diester formulation was more bioavailable than the free lutein formulation for most individuals studied. Although these data do not represent true bioavailability, which requires the calculation of AUC ratios from both alimentary and vascular administration (and an isotope label for lutein), the design, sampling times and calculations follow the U.S. Food and Drug Administration and European Union guidelines (24). The evaluation of the bioavailability of normal dietary constituents such as lutein is problematic because most tissues contain preexisting lutein stores and, unless restricted, the diet will also contain lutein. If dietary lutein is restricted during the experiment, the underlying serum lutein concentrations (originating from diet and tissues) will decrease as we observed for both lutein (Fig. 1) and other dietary carotenoids (data not shown). We sought to overcome the effect of the underlying decrement in lutein concentrations on AUC by including the entire lutein concentration (underlying plus dose dependent) in the calculation, which produces larger AUC and minimizes the variances that may have been caused by endogenous lutein stores, and by estimating the fasting lutein concentration decrement and subtracting these values from the observed lutein concentrations to obtain circulating lutein values attributable to the given dose. The lutein diester formulation was more bioavailable than the unesterified lutein regardless of the procedure used for estimating AUC in this single-dose study. Multiple-dose studies would be required with particular formulations to properly characterize lutein accumulation in target tissues.
Before exploring the possible reasons for greater bioavailability of the lutein diester formulation, it is illuminating to compare our baseline corrected data with those of Kostic et al. (17), which are included in Table 4. Both studies used the same study design and dose, and the human subject characteristics were similar. Baseline serum lutein concentrations for the two studies were almost identical. Kostic et al. (17) used the same free lutein formulation (Kemin), but extracted it with ethyl acetate, evaporated it completely, dissolved it in tocopherol-stripped corn oil and obtained a clear corn oil solution at 3.84 mmol lutein/L. To provide 0.5 μmol lutein/kg body, subjects consumed 0.13 mL corn oil/kg body, which would have been ∼10 and 8 mL for men and women, respectively. We decided to use each formulation as it might be provided in a dietary supplement. The lutein formulation was a paste containing 20 g/100 g lutein and 80 g/100 g of safflower oil, whereas the lutein diester formulation was a powder, and was ∼50 g/100 g lutein. Both formulations were packed directly into gelatin capsules. The fat and energy contents of subjects' breakfasts in the study of Kostic et al. (17) (including the oil used for dissolution) were slightly lower than those of the breakfasts consumed by our subjects. Their lutein serum concentration time curve parameters are compared in Table 4 with those found in our study. Lutein, in true solution dissolved in oil, was clearly more bioavailable than either formulation used in our study, with 61 and 160% greater mean AUC compared with the lutein diester and the unesterified lutein formulation, respectively. The time to peak of the oil-dissolved lutein was ∼50% shorter than either formulation, and maximum lutein concentrations were 335 and 691% greater than from the lutein diester or unesterified lutein used in our study, respectively. The CV for AUC were 97, 83 and 43% for lutein diester, lutein and lutein in oil solution, respectively. The lower bioavailability of these formulations, as they might be consumed as dietary supplements, was marked by greater variability in individual response.
Free lutein, although lipid-soluble, contains two hydroxyl groups at each end of the molecule. In lipid membranes, both lutein and zeaxanthin span the lipid bilayer with their hydrophilic hydroxyl groups oriented at each lipid-water interface, whereas the carotenes such as β-carotene and lycopene lie parallel to the membrane surface, deep within the hydrophobic core (25,26). One would expect free lutein to be situated on the surface of liposomes formed in the digestive process. On the other hand, lutein diesters have fatty acids, mainly palmitate, esterified to the hydroxyl groups. These large hydrophobic molecules are more likely to be situated in the core of the liposome. Furthermore, lutein diesters must be hydrolyzed to free lutein by nonspecific hydrolases either in the gut lumen or in the enterocyte because free lutein is the major form in the circulation after digestion (27). Because of these additional steps, we expected the lutein ester formulation to be slightly less bioavailable than the unesterified lutein formulation. From our data, ester hydrolysis is not the limiting step for lutein diester absorption. Rather, dissolution (dispersion, solubilization and incorporation into micelles) of each formulation as provided in capsules, seemed to be the more important factor in lutein or lutein diester bioavailability. These data indicate that the bioavailability of lutein from supplements may depend to a great extent on industrial formulation and processing of either the free lutein or the lutein diester.
Difficulties in dissolution often lead to greater variability in individual response and may explain why a few individuals responded quite differently to either the lutein diester or lutein formulations (see subjects #2, 3 and 16 vs. subjects # 1, 4 and 10 in Fig. 2). Neither dose nor gender explained the difference in response to the two formulations. To determine whether the lutein diester formulation was more bioavailable than the lutein formulation when fat intake was low, the quantity of fat in the breakfast meal (the meal most likely to influence bioavailability) was correlated with the difference in the baseline corrected AUC (lutein diester - lutein AUC) for each subject. There was an inverse correlation between difference in AUC and breakfast fat intake, r = −0.46 (R2 = 21%) that was marginally significant (P = 0.06). The higher fat breakfasts appeared to aid the dissolution of the unesterified lutein formulation compared with the lutein diester formulation, with subjects consuming ≥19 g fat more likely to efficiently “absorb” the free lutein formulation. Subjects consuming ≤11 g of fat at breakfast were more likely to fare better with the lutein diester formulation. Although it is tempting to suggest that a higher quantity of fat would slow gastric emptying and would increase the absorption of the formulation with the poorer solubility (the unesterified lutein formulation), the relatively small amount of fat in these breakfasts along with the small differences in the quantity of fat consumed among subjects would rule out differences in transit time due to fat intake as an important factor. Roodenburg et al. (28) found that the amount of fat in a meal affected lutein ester bioavailability more than that of carotenes. They supplemented 14–15 men and women per group for two 7-d periods (low fat vs. high fat) with either lutein diester, vitamin E or α- and β-carotene incorporated into low fat or high fat spreads. All subjects were counseled to consume a diet low in fat, carotenoids and vitamin E; the breakfast meal was prescribed and contained either 5 or 45 g fat, producing plasma lutein increases of 88 and 208%, respectively, in the lutein diester-supplemented group. There was no difference in the plasma increments of α- or β-carotene with the different fat intake at breakfast. Because the breakfasts in our study ranged from 11.6 to 23 g of fat, the amount of fat could have affected both lutein diester and lutein bioavailabilities. The effect that low fat meals may have on lutein or lutein diester dissolution is important because most subjects enrolled in a clinical trial may be taking their lutein supplement at breakfast. Our breakfasts, which represented typical U.S. breakfasts, may have been too low in fat for maximal absorption.
Other investigators have used lutein diester formulations over longer periods of time and have obtained substantial rises in plasma free lutein concentrations. A marigold extract containing lutein mixed esters was used to supplement 18 men and women for 4 mo, with 15 mg lutein/d (29,30). Plasma lutein concentrations rose two to sevenfold but only small amounts (<3% of total plasma lutein) of esterified lutein were found in the plasma of the highest accumulators after 1 mo of supplementation. The authors suggested that the appearance of small amounts of diester in plasma might indicate that a saturation capacity was reached for the liberation or disposition of free lutein when supplemented at high levels (29,30). We did not explore the possibility of diester appearance with either the diester or unesterified lutein formulations (which might have resolved whether circulating lutein esters are absorbed directly or produced through reesterification) because the detection of esterified lutein from a single dose of either formulation would have been below the detectable limit of measurement. Furthermore, lutein ester standards were not available. Berendschot et al. (31) supplemented 8 subjects with 10 mg/d of marigold-derived lutein diester for 10 wk, and obtained a ninefold increase in plasma free lutein concentrations and a mean linear 4-wk increase of 5.3% in macular pigment density, indicating that macular pigment density can be increased with lutein diester supplementation.
From these chronic supplementation studies and our study, it is clear that when lutein esters are consumed, substantial amounts of free lutein are released into the circulation, which is consistent with the absorption and metabolism of β-cryptoxanthin esters from tangerines. Free β-cryptoxanthin was found in plasma and chylomicrons in supplemented humans who had no detectable accumulation of its esters (32).
In conclusion, we found that the lutein diester formulation was more bioavailable than the free lutein formulation, and dissolution appeared to be more important to bioavailability than the presence or absence of esterified fatty acids. A substantial amount of the lutein appeared as free lutein in the circulation. Sufficient fat in the meal proximate to supplement consumption may be important for maximizing bioavailability. These data illustrate the importance of determining the pharmacokinetics, circumstances of ingestion and population variance for any lutein formulation selected for clinical trials.
LITERATURE CITED








 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 