Suzanne Albrecht, PharmD, MSLIS
Freelance Medical Writer
Woodstock, Illinois
4/19/2010
US Pharm. 2010;35(4):26-33.
Age-related macular degeneration (AMD) is the most common cause of blindness in the developed world.1-3 In this disease, the photoreceptors of the macula (the central retina) become damaged and die.3 AMD results in central vision loss and is responsible for one-third of all forms of untreatable loss of vision.4 An estimated 9 million older Americans have some form of AMD, and about 1.75 million have advanced AMD.5,6 AMD is a disease of the elderly, and evidence suggests that 10% of individuals aged 65 to 74 years and 30% of those aged 75 to 85 years have evidence of AMD.5 AMD is a gradual, painless, irreversible process in which the patient loses bilateral vision.1,7
AMD starts with deposits of lipid material that accumulate under the retinal pigment epithelium (RPE). These deposits, which appear as pale yellow spots on the retina, are called drusen.8 With increasing age, the RPE cells, which form the blood-retinal barrier, become less efficient and the retina is no longer able to receive the proper nutrition. This decline in the efficiency of the RPE cells also results in the accumulation of waste products (drusen).3 However, most people with evidence of drusen deposits maintain good vision.5
TYPES OF AMD
There are two forms of advanced AMD: dry (atrophic) and wet (exudative).2 The dry form is more common, but the vision loss is more severe with the wet form.9 It is thought that inflammation may play an important role in both forms of AMD.4,7 The late stages of AMD involve atrophy of the macula (which occurs in dry AMD) or growth of new blood vessels under the retina and pigment epithelium (which occurs in wet AMD).4
Dry AMD
Dry AMD is also called geographical atrophy.1 In the dry form, there is no abnormal vascularization in the subretinal space, and therefore there is no exudate.2,7 Drusen deposits are clustered on the macula, and these become larger and more numerous over time. Eventually, the RPE becomes detached and atrophies. This results in a loss of vision due to the interference of photoreceptor function.7 There is no treatment for dry AMD.4
The most common symptom of dry AMD is blurred vision, which may go away in bright light. Patients may experience difficulty with reading or recognizing faces. There may also be a blind spot in the middle of their field of vision. The blind spot may be small initially, but it can grow over time.10
Wet AMD
Wet AMD, also known as choroidal neovascularization (CNV), is less prevalent.1,7 Only about 10% of cases of AMD are the neovascular form; however, 80% to 90% of patients with severe vision loss secondary to AMD have neovascular AMD.9,11 The neovascular form involves angiogenesis and inflammation.11 Blood vessels grow from the choroid through defects in Bruch's membrane underneath the retina and pigment epithelium.2,7 These new, immature blood vessels leak lipids and blood, causing elevation of the retina and pigment epithelium.7 Irreversible damage occurs, resulting in the blurring and distortion of vision.7,11With repeated bleeding under the retina, there is permanent loss of central vision.7 Mast cells, macrophages, and lymphocytes have been found in the areas of disruption in Bruch's membrane, the CNV tissue, and the atrophied pigment epithelium. This observation strengthens the postulation that inflammation is a factor in the development of AMD.12
Neovascular AMD may be characterized as either occult or classic, based on the appearance of the CNV on fluorescein angiography. Occult CNV is usually limited to the space beneath the RPE, and the degree of vision loss is usually mild compared with classic CNV. Classic CNV often penetrates the pigment epithelium and grows into the subretinal space.13
The symptoms of neovascular AMD are usually gradual, but acute vision loss may result from bleeding of the subretinal CNV.7Symptoms of neovascular AMD include a blind spot and a distortion of vision that makes straight lines appear crooked.9,10 One or both eyes may be affected, at the same time or sequentially.9 Symptoms may develop gradually and not be noticed at first, or the patient may experience difficulty with normal activities such as reading, watching television, recognizing faces, or driving.9
RISK FACTORS
The cause of AMD is unknown, but there are factors that increase the likelihood of developing the disease (TABLE 1).2 Smoking is the only proven modifiable risk factor.1-4 Smokers are twice as likely as nonsmokers to develop AMD.1 High blood levels of homocysteine have been associated with a higher risk of developing AMD.6 Elevated homocysteine levels may be another modifiable risk factor, but this has not been clinically proven. Older age and family history are known risk factors.1,3,4,14 Individuals with a family history of AMD are four times more likely to develop the disease than those with a negative family history.5
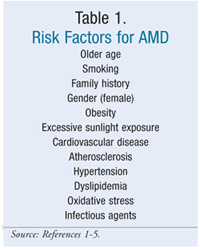
Women seem to be more likely to develop AMD than men.3 AMD has been associated with cardiovascular disease and atherosclerosis and their risk factors (hypertension and dyslipidemia).1,4,5 Other risk factors for AMD include oxidative stress, excessive sunlight exposure, and obesity.1,5 Patients with a high body-mass index (BMI) are twice as likely to develop AMD as patients with a healthy body weight.1
There is some evidence that exposure to Chlamydia pneumoniae is associated with the development and progression of AMD.4 C pneumoniae DNA has been found in the neovascular tissue of AMD-affected eyes.4 The Cardiovascular Health and Age-Related Maculopathy study examined the association between AMD and C pneumoniae exposure.4 The investigators collected serum from 254 study participants with features of early AMD (intermediate drusen, soft drusen, and pigment epithelium abnormalities) and quantified the titers of antibodies against C pneumoniae using enzyme-linked immunosorbent assay. They found that the progression rate of AMD over a 7-year period was greater in patients with higher C pneumoniae antibody titers. This observation held true even after differences in age, smoking status, family history, and history of heart attack, stroke, and/or hypertension were controlled for.4 However, in a later cohort study, the investigators failed to find an association between C pneumoniae and early AMD. The study was not powerful enough to determine whether there was an association between C pneumoniae and late AMD.12
A different study exploring the relationship between C pneumoniae and AMD was conducted. Genomic DNA was extracted from the peripheral blood of 148 subjects with advanced AMD and 162 controls. The researchers found that prior infection with C pneumoniae was associated with an increased risk of AMD.14
TREATMENT
There is no treatment for the dry form of AMD or the early stages of neovascular AMD.1,4,6 All FDA-approved treatment modalities for the neovascular form of AMD target CNV.
Photodynamic Therapy (PDT)
PDT is used to treat predominantly classic subfoveal CNV, but only about a third of patients with neovascular AMD have the classic form.9 PDT involves injecting a photosensitizing drug intravenously. This drug, called verteporfin (Visudyne, QLT), was approved by the FDA in 2006.15 Verteporfin has a high affinity for CNV.9 A low-energy laser is focused on the CNV, which activates the dye.16 The laser is then used to occlude the CNV vessels. This procedure causes little to no persistent short-term damage to the adjacent CNV or to the retina.9 More than 90% of patients who undergo PDT require retreatment after 3 months, and most patients need multiple treatments during the first year.9
Antiangiogenic Therapy
Unlike PDT, which destroys existing CNV, antiangiogenic therapy prevents further neovascularization.9 Angiogenesis is the formation of new blood vessels. It is a complex process and requires interactions between various factors, some of which are inhibitory and some of which are stimulatory. Antiangiogenic therapy works by either promoting inhibitory factors or blocking stimulatory factors.9 Vascular endothelial growth factor (VEGF) stimulates the formation of endothelial blood vessels; it also increases the permeability of CNV and acts as a vasodilator.11,16 There are many isoforms of VEGF, but VEGF-A121 and VEGF-A165 are the most prevalent in the retina.11 Moieties that inhibit VEGF prevent the growth of these vessels.9 The three FDA-approved anti-VEGF agents used in the United States are pegaptanib (Macugen, Eyetech), bevacizumab (Avastin, Genentech), and ranibizumab (Lucentis, Genentech).
Pegaptanib: This agent, which has been proven to reduce vision loss, was approved by the FDA in 2004.9 An RNA aptamer, pegaptanib binds to the VEGF-A165 isoform, which is the most predominant isoform found in the CNV.11 It is injected into the vitreous every 6 weeks.17 The most common adverse events reported with the use of pegaptanib 0.3 mg for up to 2 years are anterior chamber inflammation, blurred vision, cataract, conjunctival hemorrhage, corneal edema, eye discharge, eye irritation, eye pain, increased intraocular pressure, punctate keratitis (corneal inflammation), reduced visual acuity (VA), visual disturbances, vitreous floaters, and vitreous opacities. These events occurred in approximately 10% to 40% of patients.17 The most serious adverse events are endophthalmitis (inflammation of the ocular cavities), traumatic injury to the lens, and retinal detachment.11
Bevacizumab: Bevacizumab is a monoclonal antibody that is three times as large as ranibizumab. Approved in 2004 for the treatment of metastatic colon cancer, it has since been approved, as monotherapy or in combination with other agents, for the treatment of nonsquamous non-small cell lung cancer, metastatic breast cancer, glioblastoma, and metastatic renal cell carcinoma.11,18 Bevacizumab inhibits all of the known isoforms of VEGF-A, and it is used off-label to treat wet AMD and macular edema. The agent is administered intravitreously, and in several uncontrolled studies it has been shown to be safe and effective against wet AMD. It is less expensive than ranibizumab and is often used as an alternative when cost is an issue.11
Ranibizumab: This agent, another monoclonal antibody, was approved in 2006 for the treatment of wet AMD and is active against all forms of VEGF-A.11 The recommended dose is 0.5 mg injected intravitreously once a month; however, the dosing interval may be increased to once every 3 months after the first four injections. The longer dosing interval is not as effective as the monthly interval, but it may be necessary if cost is prohibitive or if monthly injections are not tolerated.19 More than one-third of patients using ranibizumab have experienced improvement of vision, and vision loss has been prevented in 95% of patients using ranibizumab over a 2-year period.20 No other treatment for AMD has come close to this success, with the possible exception of bevacizumab.16
The ANCHOR study was a multicenter (83 sites), double-blind, active-treatment, controlled, phase III trial comparing the efficacy and adverse-event profile of ranibizumab versus PDT in treating patients with predominantly classic subfoveal CNV.13 At least 50% of a patient's lesions had to be the classic type, and only one eye per patient was treated. Patients received either verteporfin PDT and a sham injection or sham verteporfin PDT and a monthly intravitreous injection of ranibizumab (either 0.3 mg or 0.5 mg). In the active-PDT arm, 110 patients completed the study; active ranibizumab treatment was completed by 117 patients and 116 patients, respectively, receiving 0.3 mg or 0.5 mg. CNV lesions were examined by fluorescein angiography every 3 months. VA was measured at baseline, 12 months, and 24 months with Early Treatment Diabetic Study charts at a starting distance of 2 m and using standardized refraction.
Ranibizumab's superiority over PDT was evident at 1 month.13 At 24 months, 90.0% of patients in the 0.3-mg ranibizumab group and 89.9% of patients in the 0.5-mg ranibizumab group had lost <15 letters from baseline VA, whereas only 65.7% of PTD patients lost <15 letters. Furthermore, a gain of ≥15 letters was observed in 34.3% of the 0.3-mg ranibizumab group and 41.0% of the 0.5-mg ranibizumab group. Only 6.3% of PDT patients gained ≥15 letters. At 24 months, 60.8% of PDT patients versus 22.9% of the 0.3-mg ranibizumab group and 20% of the 0.5-mg ranibizumab group demonstrated a VA Snellen equivalent of ≥20/200 (P <.0001). At 24 months, severe vision loss (loss of ≥30 letters) was apparent in only 1.4% of the 0.3-mg ranibizumab group and in none of the 0.5-mg ranibizumab group versus 16.1% of PDT patients.
No other treatment to date has been able to improve VA as well as preserve it. Serious adverse events have occurred in less than 0.1% of patients and include retinal detachment, endophthalmitis, and iatrogenic traumatic cataracts.19
ANTIOXIDANTS
Because oxidative stress is thought be involved in the development of AMD, many studies have been conducted to assess the role antioxidants may play in the prevention or treatment of AMD.2 The Age-Related Eye Disease Study (AREDS), a landmark study sponsored by the National Eye Institute, was a multicenter (11 sites), longitudinal, double-blind, placebo-controlled, clinical trial examining the effect of high doses of vitamins C and E, beta carotene, and zinc on the progression of AMD.21 Patients had to have extensive small, intermediate, or large drusen, noncentral geographical atrophy, or pigment abnormalities in one or both eyes or advanced AMD or vision loss secondary to AMD in one eye. Patients, aged 55 to 80 years, were randomized to receive a daily oral dose of (1) the antioxidants vitamin C (500 mg), vitamin E (400 IU), and beta carotene (15 mg); (2) zinc (80 mg zinc oxide) and copper (2 mg cupric oxide); (3) antioxidants plus zinc; or (4) placebo. Primary outcomes were progression to advanced AMD and VA loss of ≥15 letters. Patients were assigned to one of four AMD categories based on the size and extent of drusen and RPE abnormalities in each eye, the presence of advanced AMD, and VA, with category 1 being least severe and category 4 being most severe.
The risk of progression to advanced AMD in AMD categories 3 and 4 was reduced by 17% in patients taking only antioxidants, 21% in patients taking only zinc, and 25% in patients taking both antioxidants and zinc.21 There were too few cases of advanced AMD in category 2 to assess whether treatment affected progression of AMD in that category. Only category 3 and 4 patients in the antioxidants-plus-zinc arm had a reduction in VA loss of ≥15 letters (27%; P = .008).
Based on these results, the AREDS research group suggests that everyone over 55 years of age should have dilated eye examinations. Patients with extensive intermediate-size drusen, ≥1 large druse, noncentral geographical atrophy in one or both eyes, or advanced AMD or vision loss secondary to AMD should consider taking the antioxidants-plus-zinc formulation used in this study. This recommendation excludes individuals with contraindications such as smoking.21 Bausch & Lomb, which collaborated in AREDS, currently markets PreserVision, a supplement based on the formula used in the AREDS.22
Other natural supplements, including lutein and zeaxanthin, have shown some evidence of reducing the risk of AMD development.3One study suggested a 35% to 40% decrease in the risk of developing AMD in women aged 40 years and older who supplemented their diet with folic acid (2.5 mg/day), pyridoxine (50 mg/day), and cyanocobalamin (1 mg/day).6
CONCLUSION
AMD, which affects primarily older people, is a debilitating disease that can lead to blindness. The only proven modifiable risk factor for developing AMD is smoking, but other factors, such as genetics, elevated BMI, presence of cardiovascular disease, hypertension, female gender, and exposure to certain infectious agents, also may increase the risk. Currently, only wet AMD is treatable. PDT and antiangiogenic pharmaceuticals are the only options available at present, but there are other compounds in the drug-development pipeline that may aid in the battle against AMD.10 PreserVision, a Bausch & Lomb product, is based on the supplement formulation used in the AREDS.
Since endophthalmitis may develop following antiangiogenic therapy, the pharmacist should instruct these patients to contact their ophthalmologist immediately if they notice eye redness or pain, sensitivity to light, or vision changes.17,19 The pharmacist should advise patients who have just undergone PDT that they will experience temporary sensitivity to light. For 5 days, patients should avoid exposing unprotected skin to direct sunlight and bright indoor light, including tanning salons, bright halogen lighting, and high-power lighting such as that used in operating rooms or dental offices. During the first 5 days, patients should wear dark glasses and protective clothing. Ultraviolet sunscreens are not effective protection because photoactivation of verteporfin in the skin can be caused by visible light. Patients should be instructed not to stay in the dark, but rather to expose their skin to indoor light; this will help inactivate the drug through photobleaching.23
The pharmacist is in a key position to counsel patients about risk factors for AMD. In addition, the pharmacist can be a valuable resource for informing patients about the symptoms of AMD and the utility of antioxidants plus zinc for reducing their risk of developing advanced AMD.
REFERENCES
1. Baird PN, Hageman GS, Guymer Franzco RH. New era for personalized medicine: the diagnosis and management of age-related macular degeneration. Clin Experiment Ophthalmol. 2009;37:814-821.
2. Katzung BG. Special aspects of geriatric pharmacology. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill Medical; 2009:1037-1045.
3. Carpentier S, Knaus M, Suh M. Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr. 2009;49:313-326.
4. Robman L, Mahdi O, McCarty C, Dimitrov P, et al. Exposure to Chlamydia pneumoniae infection and progression of age-related macular degeneration. Am J Epidemiol. 2005;161:1013-1019.
5. Phillips CO, Higginbotham EJ. Multivitamin supplements, ageing, and loss of vision: seeing through the shadows. Arch Intern Med. 2009;169:1180-1182.
6. Christen WG, Glynn RJ, Chew EY, et al. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women's Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med. 2009;169:335-341.
7. Horton JC. Disorders of the eye. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison's Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008.
8. Evans JR, Henshaw KS. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. 2008;(1):CD000253.
9. Vedula SS, Krzystolik MG. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2008;(2):CD005139.
10. National Eye Institute. Facts about age-related macular degeneration. www.nei.nih.gov/health/maculardegen/armd_facts.asp#1c. Accessed February 18, 2010.
11. Ni Z, Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica.2009;223:401-410.
12. Robman L, Mahdi OS, Wang JJ, et al. Exposure to Chlamydia pneumoniae infection and age-related macular degeneration: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2007;48:4007-4011.13. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two year results of the ANCHOR study. Ophthalmology. 2009;116:57-65.
14. Shen D, Tuo J, Patel M, et al. Chlamydia pneumoniae infection, complement factor H variants, and age-related macular degeneration. Br J Ophthalmol. 2009;93:405-408.
15. Visudyne.com. Welcome to the Visudyne® AMD resource center. www.visudyne.com. Accessed January 30, 2010.
16. Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology.2009;116(suppl 10):S15-S23.
17. Macugen (pegaptanib sodium injection) package insert. Cedar Knolls, NJ: Eyetech Inc; August 2008.
18. Avastin (bevacizumab) package insert. South San Francisco, CA: Genentech Inc; July 2009.
19. Lucentis (ranibizumab injection) package insert. South San Francisco, CA: Genentech Inc; April 2008.
20. Do DV. Antiangiogenic approaches to age-related macular degeneration in the future. Ophthalmology.2009;116(suppl 10):S24-S26.
21. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436.
22. Bausch & Lomb. PreserVision® Eye Vitamin AREDS Soft Gel formula. www.bausch.com/en_US/consumer/visioncare/product/vitamins/preservision_softgel.aspx. Accessed February 17, 2010.
23. Visudyne (verteporfin for injection) package insert. Vancouver, Canada: QLT Inc; January 2010.
其他跟黃斑部病變有關係的文章:點我
或是你想要再一次了解眼睛的構造:眼睛的解剖圖





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 