JAMA. 2014;311(16):1670-1683. doi:10.1001/jama.2014.3654
ABSTRACT
Importance Parkinson disease is the second most common neurodegenerative disease worldwide. Although no available therapies alter the underlying neurodegenerative process, symptomatic therapies can improve patient quality of life.
Objective To provide an evidence-based review of the initial pharmacological management of the classic motor symptoms of Parkinson disease; describe management of medication-related motor complications (such as motor fluctuations and dyskinesia), and other medication adverse effects (nausea, psychosis, and impulse control disorders and related behaviors); and discuss the management of selected nonmotor symptoms of Parkinson disease, including rapid eye movement sleep behavior disorder, cognitive impairment, depression, orthostatic hypotension, and sialorrhea.
Evidence Review References were identified using searches of PubMed between January 1985 and February 2014 for English-language human studies and the full database of the Cochrane Library. The classification of studies by quality (classes I-IV) was assessed using the levels of evidence guidelines from the American Academy of Neurology and the highest-quality data for each topic.
Results Although levodopa is the most effective medication available for treating the motor symptoms of Parkinson disease, in certain instances (eg, mild symptoms, tremor as the only or most prominent symptom, aged <60 years) other medications (eg, monoamine oxidase type B inhibitors [MAOBIs], amantadine, anticholinergics, β-blockers, or dopamine agonists) may be initiated first to avoid levodopa-related motor complications. Motor fluctuations may be managed by modifying the levodopa dosing regimen or by adding several other medications, such as MAOBIs, catechol-O-methyltransferase inhibitors, or dopamine agonists. Impulse control disorders are typically managed by reducing or withdrawing dopaminergic medication, particularly dopamine agonists. Evidence-based management of some nonmotor symptoms is limited by a paucity of high-quality positive studies.
Conclusions and Relevance Strong evidence supports using levodopa and dopamine agonists for motor symptoms at all stages of Parkinson disease. Dopamine agonists and drugs that block dopamine metabolism are effective for motor fluctuations and clozapine is effective for hallucinations. Cholinesterase inhibitors may improve symptoms of dementia and antidepressants and pramipexole may improve depression. Evidence supporting other therapies for motor and nonmotor features is less well established.
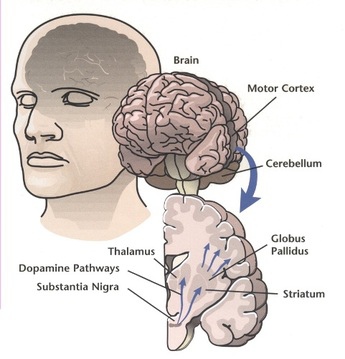
Loss of dopamine-secreting neurons within the substantia nigra and presence of Lewy bodies are the major pathological findings in Parkinson disease. Early in the disease course, dopamine deficiency is the predominant neurochemical abnormality. As the disease progresses, involvement of nondopaminergic brain regions results in levodopa-resistant motor and nonmotor symptoms.
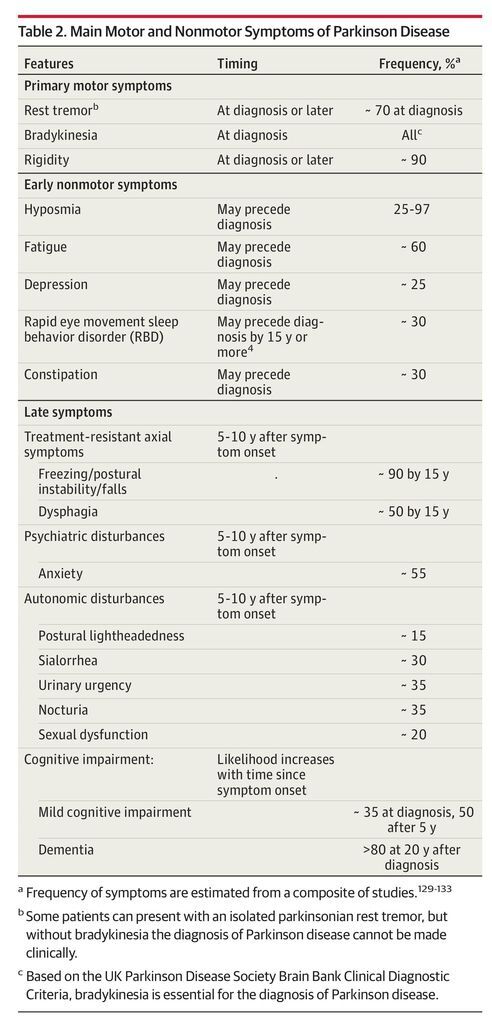
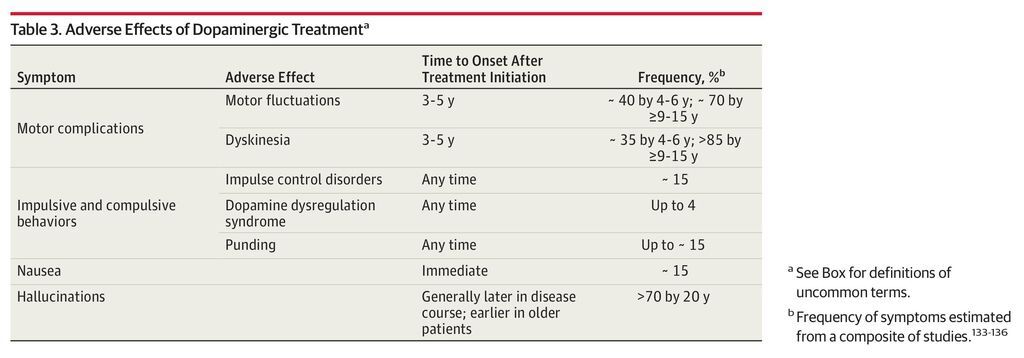
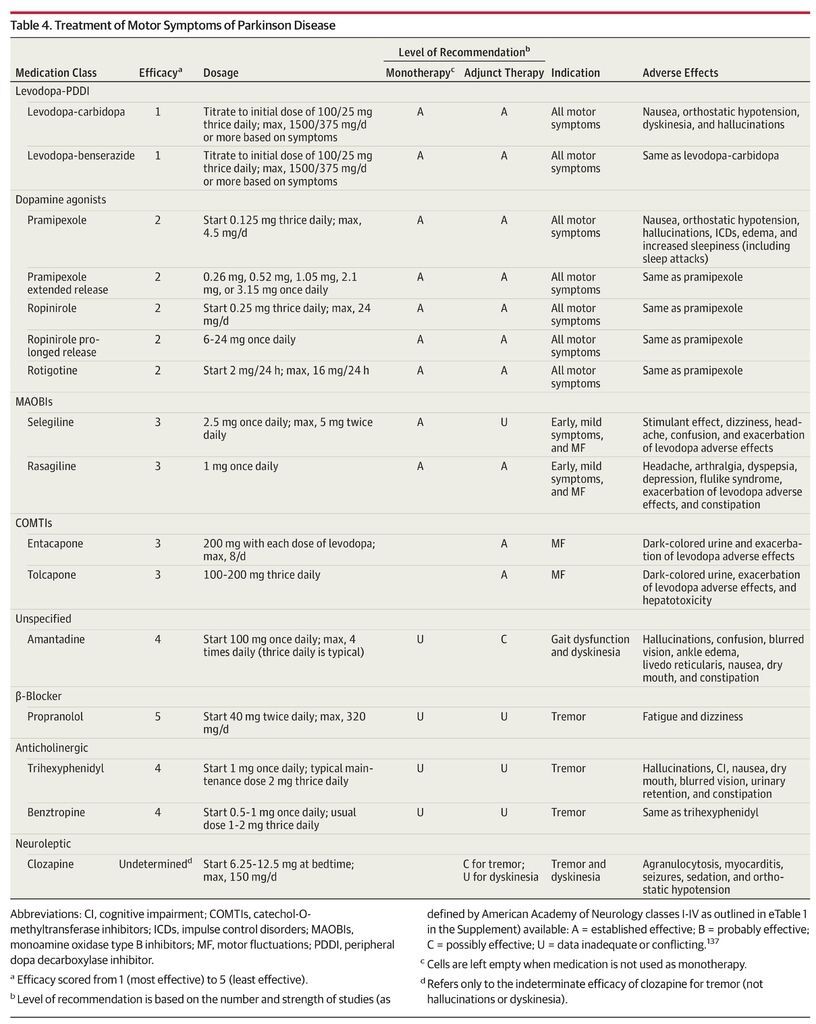
Although Parkinson disease is incurable, therapies can improve quality of life for many years. We discuss the pharmacological management of important motor and nonmotor features of Parkinson disease (Table 2) and adverse effects of therapy (Table 3). The Box provides definitions for commonly used terms.
-
“On” period: Periods when the patient experiences a good response to medication
-
“Off” period: Periods when benefit from Parkinson disease medications wears off and symptoms reemerge (wearing-off–type motor fluctuations)
-
Motor fluctuations: Alterations between on and off periods
-
Dyskinesia: Involuntary, nonrhythmic choreic or choreo-dystonic movements most often related to peak dopamine levels; they can lead to social embarrassment, impaired motor function, injury, and weight loss
-
Rapid eye movement (REM) sleep behavior disorder: Characterized by the loss of muscle atonia during REM sleep resulting in acting out of dreams, often with aggressive or violent behavior, sometimes resulting in injury to the patient or his/her bed partner
-
Impulse control disorders: Include pathological gambling, hypersexuality, binge eating, and compulsive shopping
-
Dopamine dysregulation syndrome: A form of addictive behavior with the compulsive overuse of dopaminergic medications (typically shorter-acting medications, such as levodopa and apomorphine) impairing physical, social, and occupational functioning5
-
Punding: Repetitive, often purposeless, stereotyped behaviors, such as sorting or disassembling6
METHODS
Search
References were identified using searches of PubMed between January 1985 and February 2014 for English-language human studies and the full database of the Cochrane Library. The search strategy and search results are provided in the eAppendix in the Supplement.
Study Classification
Studies were classified using the American Academy of Neurology (AAN) Classification Scheme Requirements for Therapeutic Questions and rated on quality of evidence: class I (CI) and class II (CII) relate to randomized clinical trials (RCTs), class III (CIII) indicates other controlled trials, and class IV (CIV) indicates other7 (eTable 1 in the Supplement). The AAN Classification of Recommendations guidelines8 (eTable 2 in the Supplement) were used to provide a level of recommendation (A = established effective; B = probably effective; C = possibly effective; U = data inadequate or conflicting) for each therapy. Randomized clinical trials were arbitrarily classified by size: those with 50 or fewer participants were considered small, those with 51 to 200 were intermediate, and those with more than 200 participants were designated as large.
Study Inclusion
Meta-analyses and individual RCTs fulfilling criteria for the highest quality of evidence (CI and CII) were primarily included, but if few or none were identified for a topic, then lower-quality evidence was reviewed. Published guidelines for management of Parkinson disease were also included. All nonpharmacological studies and studies on ergot dopamine agonists (bromocriptine, cabergoline, lisuride, and dihydroergocryptine) and piribedil (unavailable in North America) were excluded.
RESULTS
Initiation of Therapy for Motor Symptoms in Early Parkinson Disease
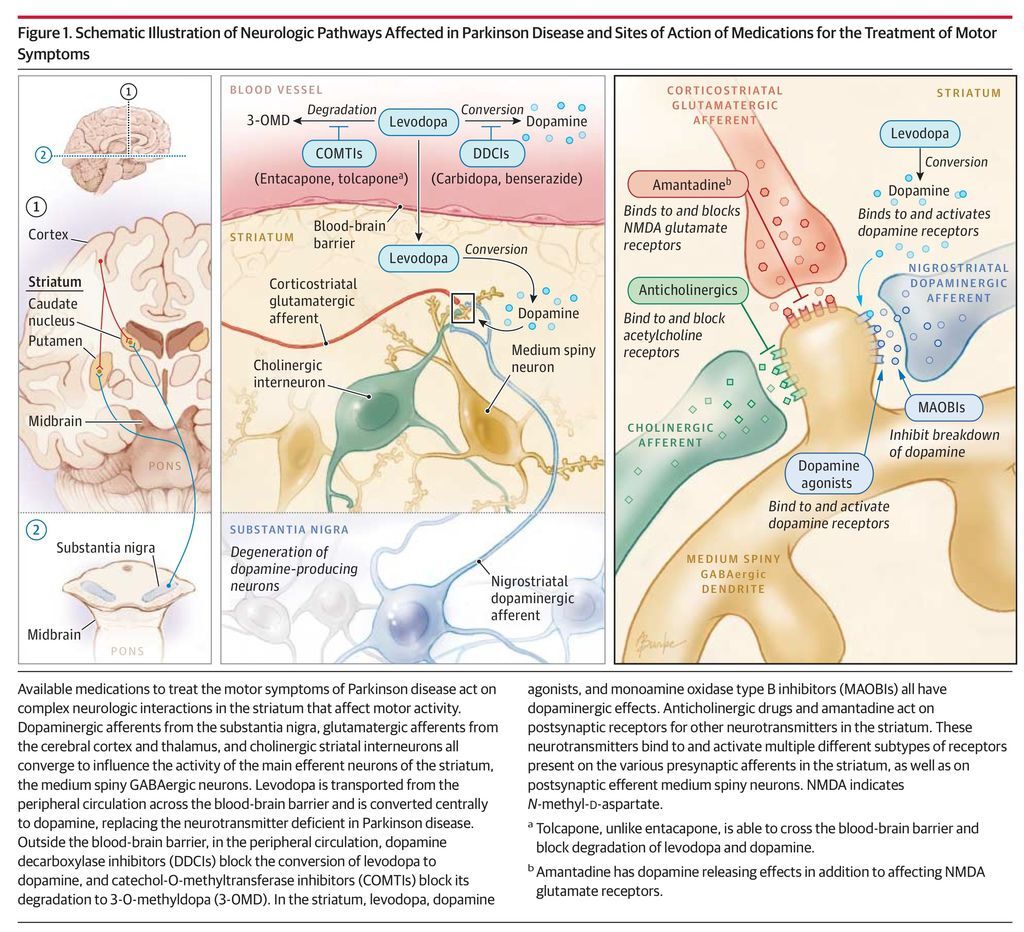
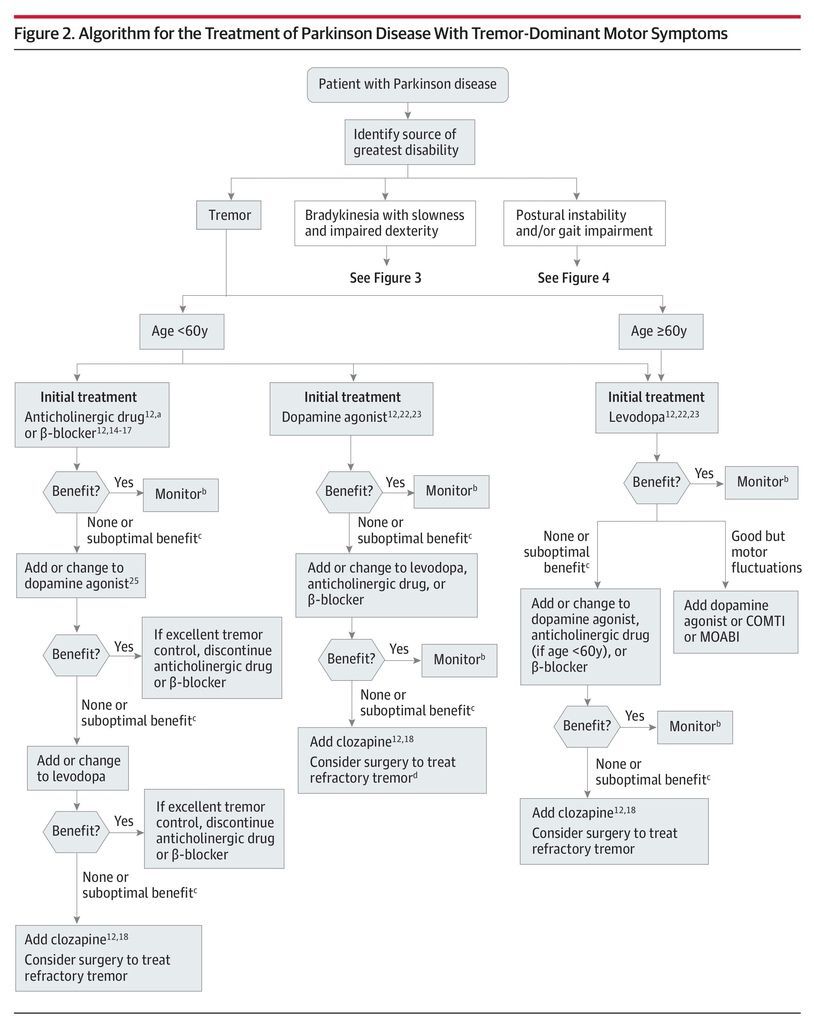
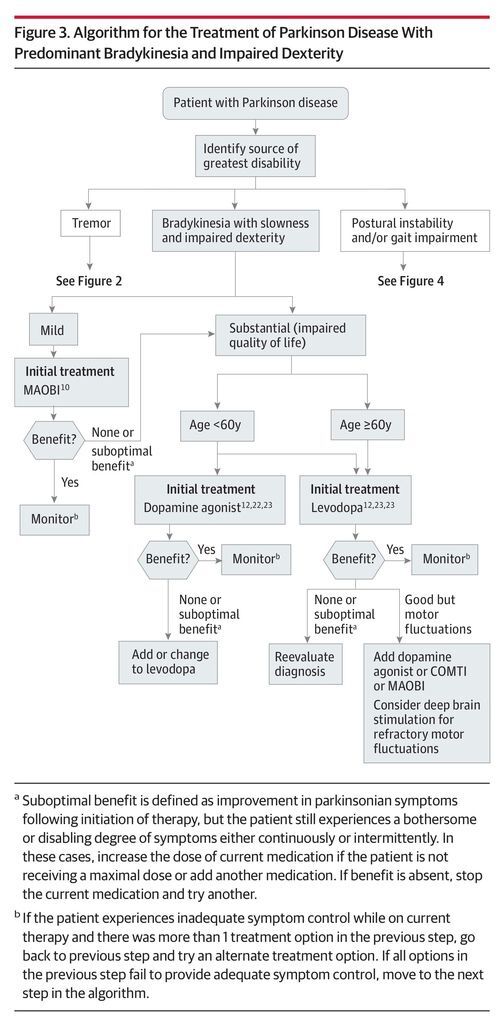
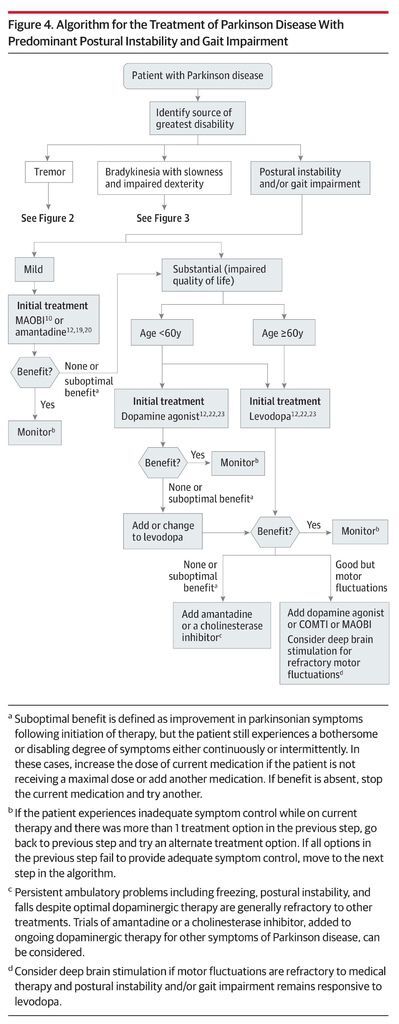
There are no established disease-modifying or neuroprotective therapies.9 Medication should be initiated when patients experience functional impairment or social embarrassment from their symptoms. Figure 1 illustrates mechanisms of action of available medications. Initial therapy selection typically depends on a patient’s specific symptoms and age (Table 4; Figure 2, Figure 3, Figure 4).
If motor symptoms are mild but require therapy, before moving to more potent treatment such as a dopamine agonist or levodopa, a monoamine oxidase type B inhibitor (MAOBI; selegiline or rasagiline) may be tried. A meta-analysis of MAOBIs in early Parkinson disease demonstrated a small symptomatic benefit.10 A potential disease-modifying effect of rasagiline (1 mg-dose) has been reported11 (1 large CI study) but not confirmed by additional clinical trial evidence.
A 2003 Cochrane review involving 9 heterogeneous studies determined that anticholinergic medications are more effective than placebo for improving motor function in Parkinson disease, but data on their benefits for tremor (generally considered their main indication) were inconclusive.12,13 Lower-quality, older studies suggest that β-blockers may improve parkinsonian tremor and motor function. Propranolol is used most often.12,14- 17 Clozapine has been shown to improve Parkinson disease tremor (1 CII, 2 CIII, and 2 CIV studies)12,18; this is used exclusively for bothersome or disabling tremor resistant to other therapies.
Evidence supporting amantadine for treatment of Parkinson disease (6 small CIII RCTs) is mixed. A 2009 Cochrane review concluded that there is insufficient evidence for its efficacy due to poor study quality,19 whereas expert opinion from the International Parkinson and Movement Disorder Society and European Federation of Neurological Societies concluded that amantadine is likely efficacious for symptomatic monotherapy and adjunct therapy.12,20
For those with more severely impaired activities of daily living, levodopa or a dopamine agonist is usually initiated. Multiple large CI trials demonstrate that levodopa provides the greatest symptomatic benefit for Parkinson disease12 and is associated with less freezing, somnolence, edema, hallucinations, and risk of impulse control disorders (ICDs) than dopamine agonists. However, dopamine agonists are also efficacious in early Parkinson disease21 and are less likely than levodopa to cause dopaminergic motor complications, particularly dyskinesia.22,23 Because younger age-of-onset of Parkinson disease is a risk factor for dyskinesia,24 dopamine agonists are usually introduced as initial treatment for patients younger than 60 years. However, there is increasing evidence in open-label, observational, naturalistic follow-up studies that the early advantage of dopamine agonists over levodopa diminishes over time (approximately 10 years).25- 27
Our experience supports the approaches outlined above and in Figure 2, Figure 3, and Figure 4. In patients with mild symptoms, we begin with a lower potency medication, such as an MAOBI, which may have a milder adverse effect profile and often a less frequent dosing regimen. Serotonin syndrome is a theoretical risk when using MAOBIs concurrently with another serotonergic medication. As Parkinson disease progresses, medication should be adjusted to obtain optimal symptom control. In addition to the diminishing benefits of initial dopamine agonist therapy in delaying dyskinesia after age 60, greater predisposition to cognitive and psychiatric adverse effects with age also influences treatment choices. Elderly patients, especially those with preexisting cognitive dysfunction, are at greater risk of developing psychiatric adverse effects. Thus, in older patients, the risk to benefit ratio favors levodopa compared with dopamine agonists and alternative therapies.
Managing Motor Fluctuations
Managing Symptom Reemergence Between Medication Doses
Strategies for reducing the time that medication is not optimally effective (“off” time) include increasing the dosage of dopaminergic medication, adding another dopaminergic medication, dividing the levodopa dosage into smaller but more frequent doses (levodopa dose fractionation), or adding a catechol-O-methyltransferase inhibitor (COMTI) or MAOBI to inhibit the breakdown of levodopa and dopamine and prolong their effects.28 Few trials have compared these therapeutic options. Rasagiline (an MAOBI) and entacapone (a COMTI) were not significantly different in reducing off time in 1 large CI study,29 and a subsequent substudy provided evidence that adjunct rasagiline is more effective than entacapone at reducing the severity of motor symptoms in the off time.30 A CII, open-label trial showed no significant difference in reduced off time, rate of motor complications, or daily levodopa dose with entacapone compared with levodopa dose fractionation.31
In most available CI (2 large) and CII (2 large and 3 intermediate) studies, entacapone and tolcapone (COMTIs) significantly reduced off time compared with placebo and baseline and had similar adverse effects, such as nausea, diarrhea, orthostatic hypotension, and dyskinesia.28,32,33 These 2 COMTIs have not been directly compared, but a “switching” study34 (a double-blind study in which patients taking entacapone were randomized to stay on entacapone or switch to tolcapone) and uncontrolled evidence support greater efficacy of tolcapone.35,36 However, hepatic monitoring is necessary due to rare cases of fatal hepatotoxicity with tolcapone.37
There is extensive, conflicting evidence for use of selegiline (a MAOBI) to reduce off time.21,38 Orally disintegrating selegiline significantly reduced off time compared with placebo in 1 intermediate CII study,39 but not in an intermediate CI study.40 Adjunct rasagiline decreased daily off time in 2 large CI studies.29,41
Dopamine agonists may be added to levodopa to reduce off time. Adjunctive pramipexole, ropinirole (including their extended- and prolonged-release formulations), and transdermal rotigotine (24-hour continuous delivery) significantly reduced off time compared with placebo (in at least 1 large CI study each and several intermediate CII studies)20,21,28. Prolonged-release ropinirole was more efficacious in maintaining a 20% or more reduction in off time compared with immediate-release ropinirole (1 large CI study)42 and there was no significant difference in benefit between rotigotine and pramipexole for the outcome of wearing-off–type motor fluctuations (1 large CI study).43 Intermittent, as needed, subcutaneous apomorphine provides rapid delivery to reduce bothersome off periods (1 small CII study), but may increase dyskinesia.28 A retrospective review of patients receiving continuous subcutaneous apomorphine infusion also demonstrated a significant reduction in off time compared with the patients’ baseline.44
Controlled-release levodopa-carbidopa does not reduce off time more than immediate-release levodopa-carbidopa (4 small CIII trials).28 However, a new extended-release, levodopa-carbidopa formulation reduced off time compared with the immediate-release formulation in 1 large CI study.45 An intermediate CI study of levodopa-carbidopa intestinal gel (LCIG), administered directly into the duodenum by pump through a gastrostomy catheter, confirmed early, small, open-label studies showing a marked reduction in off time.21,46,47
Management of Dyskinesia
It is unnecessary to treat mild nontroublesome dyskinesia. Dopaminergic medication reduction strategies will reduce dyskinesia but typically worsen parkinsonism.48 Amantadine is frequently used for reducing dyskinesia severity and duration (1 small CI, 1 small CII, and several lower-quality studies),28,49- 51 and is usually well tolerated. Clozapine has been shown to improve dyskinesia (1 small CIII study).21 Levodopa-carbidopa intestinal gel may be useful in the future, and exploratory trials of other agents are ongoing.
In our experience, levodopa-related wearing-off–type motor fluctuations respond, to a variable extent, to all of the reviewed therapeutic options, and, when 1 is suboptimally effective, 1 or more alternatives may be combined with greater success. Some patients require a combination of 3 or 4 different types of medications. Amantadine can be extremely effective in managing dyskinesia and efficacy is generally retained in the long-term despite common concerns about tachyphylaxis.52 It is important to regularly reevaluate the ongoing efficacy and need for various drugs, although levodopa remains the mainstay of treatment for all patients. We do not routinely use controlled-release levodopa-carbidopa and usually discontinue this in patients with motor complications given its unreliable pharmacokinetics, although when administered at bedtime it may provide better overnight Parkinson disease symptom control when nighttime symptoms are problematic. Levodopa-carbidopa intestinal gel appears promising in the management of carefully selected patients with disabling motor fluctuations.
Management of Other Medication Adverse Effects
Nausea
Nausea is a frequent, generally transient adverse effect of dopaminergic therapy. Slow titration of dopaminergic therapy and medication administration with food can reduce nausea. However, in the long-term, food may delay gastric emptying, and dietary protein may interfere with levodopa absorption.53 The dopa decarboxylase inhibitors carbidopa and benserazide prevent peripheral conversion of levodopa to dopamine. An additional dose of carbidopa 30 minutes before the regular levodopa preparation may abolish levodopa-induced (but not dopamine agonist–induced) nausea.54 Domperidone, a peripheral dopamine D2-receptor antagonist (unavailable in the United States), reduced nausea from dopaminergic medications in several small CIII and CIV studies.55,56 Trimethobenzamide has also been used for the same purpose.57 Metoclopramide, prochlorperazine, and promethazine can worsen parkinsonian symptoms and should be avoided.
Impulsive and Compulsive Behaviors Including ICDs, Dopamine Dysregulation Syndrome, and Punding
Impulse control disorders are typically, but not exclusively, associated with dopamine agonist use.58 A history of obsessive-compulsive disorder, impulsive personality, or addictive behaviors increases the likelihood of ICDs.59 Given the effect of ICDs, these need to be discussed on initiation of a dopamine agonist and monitored regularly. Dopamine agonist dose reduction or discontinuation, potentially offset by increasing levodopa, is a generally effective treatment of ICDs.60- 62 Withdrawal symptoms including anxiety, depression, fatigue, pain, orthostatic hypotension, and drug cravings (the dopamine agonist withdrawal syndrome) unresponsive to increasing levodopa may occur in 15% to 20% of patients.63
Zonisamide markedly reduced the severity of impulsive behaviors and global impulsiveness refractory to dopaminergic medication dose reduction in a small CIII study.64 Amantadine improved pathological gambling resistant to dopamine agonist dose reduction or withdrawal or behavioral strategies in 1 small CII study.65 In small case series (1 CIV study each), topiramate66 and valproate67 were effective.
Management of dopamine dysregulation syndrome (DDS) typically involves a gradual reduction of levodopa and immediate discontinuation of “booster” doses of medications (such as subcutaneous apomorphine boluses and rapid-acting levodopa formulations).5 No studies have formally examined management of DDS, but in a small case series of 4 patients (1 CIV study), all 4 responded to valproate.68
Improvement of punding may follow reduction or cessation of dopaminergic medication; amantadine and quetiapine may also be beneficial (1 small open-label, prospective, CIII study).69
Psychosis
Hallucinations are both a feature of later-stage Parkinson disease and a consequence of Parkinson disease medication, whereas additional psychotic symptoms are generally drug-related. Clozapine and quetiapine have been most extensively studied for the treatment of psychosis in Parkinson disease given the propensity of other neuroleptics to worsen parkinsonism. Clozapine is consistently efficacious (1 intermediate CI study and 1 intermediate CII study [both with open-label extensions of the study treatment use] and 2 comparative studies [small CII and CIII]), whereas results for quetiapine are mixed (4 negative blinded RCTs [1 small CI, 2 small CII, and 1 intermediate CII] and 1 positive blinded RCT [small CII], and varied results in comparative studies with clozapine).12,70- 73 However, quetiapine is often prescribed first because of the risk of agranulocytosis and the requirement for frequent blood monitoring with clozapine.
Olanzapine was found in 2 CII studies (1 small and 1 intermediate) to be ineffective for hallucinations and may cause motor deterioration,72 and another study was stopped early for these reasons74; therefore, olanzapine should not be used for Parkinson disease.72 Similar motor deterioration has been observed with risperidone and aripiprazole.75
The cholinesterase inhibitor donepezil reduced hallucinations in patients with Parkinson disease without dementia (1 small CIII study),76 and rivastigmine reduced hallucinations in those with dementia (1 intermediate CII study).77 Ziprasidone was effective for psychosis in Parkinson disease in 2 small CIII studies.78,79
In our experience, the treatment options outlined above will almost always adequately control gastrointestinal intolerance and it is extremely rare for gastrointestinal symptoms to limit the use of therapeutically effective doses of levodopa. Most problematic ICDs can be successfully managed. Involvement of caregivers, family, and friends to limit the patient’s access to money, Internet sites, food, or casinos, is often helpful. In some patients, ICDs are refractory, especially when symptoms of dopamine agonist withdrawal syndrome occur. We do not use amantadine for pathological gambling because ICDs may be more common in patients taking this drug, and we have seen them develop as a consequence of adding amantadine to levodopa. Management of DDS is extremely challenging and an expert in addiction management should assist in the care of these patients. Hospital admission may be required if prominent mood symptoms, hypomania, or psychotic episodes develop. In many patients, punding behavior can be monitored without treatment; however, when symptoms become disruptive and the patient is only taking levodopa in a dose required to maintain motor control, treatment is often unsatisfactory.
Our approach to new-onset or increased hallucinations includes initial exclusion of systemic illness (such as infection) or other medication use.80 We then reduce or discontinue antiparkinsonian drugs in order from lowest efficacy, starting with anticholinergics, amantadine, and MAOBIs, followed by dopamine agonists and COMTIs.81 Finally, the levodopa dose is cautiously reduced and the patient is monitored for a disabling increase in parkinsonism, including the rare occurrence of a neuroleptic malignantlike state. Quetiapine and clozapine can help to avoid these outcomes; clozapine clearly has the highest efficacy for hallucinations and psychosis. Cholinesterase inhibitors may reduce hallucinations, but generally not other psychotic symptoms. A very recent intermediate CI study showing efficacy for the selective serotonin 5-HT2A inverse agonist pimavanserin for psychotic symptoms82 provides new promise for future therapies not requiring extensive monitoring.
Management of Selected Nonmotor Symptoms
Rapid Eye Movement Sleep Behavior Disorder
Clonazepam is a first-line therapy for rapid eye movement sleep behavior disorder (RBD),83 but only case reports and case series are available on its use in Parkinson disease patients.84 Similarly, there is little evidence for melatonin specifically in patients with Parkinson disease, but 1 small CI RCT showed benefit in patients with RBD who were not diagnosed with Parkinson disease.84,85 Rivastigmine improved the frequency of RBD episodes in 1 small CI study.86
We prescribe melatonin for patients who do not tolerate clonazepam or as a first-line therapy for patients with relative contraindications to clonazepam (such as dementia, obstructive sleep apnea, and extreme frailty with an increased risk of falls).84
Depression
The literature on treatment of depression in Parkinson disease is mixed. A 2013 systematic review and meta-analysis assessed 6 RCTs (2 small CI, 1 intermediate CI, 1 intermediate CII, 2 small CIII) of antidepressants for depression in patients with Parkinson disease (involving sertraline, citalopram, paroxetine, venlafaxine, desipramine, and nortriptyline).87 There was no statistically significant superiority of antidepressants compared with placebo, as a group or by class, but tricyclic antidepressants were superior to selective serotonin receptor inhibitors (SSRIs; 2 studies88,89). However, a sensitivity analysis that excluded studies with questionable results, high risk of bias (3 of 6 studies), or both found antidepressants to be efficacious. Two other recent meta-analyses showed no significant pooled effect of antidepressants and insufficient evidence to support use of SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), or pramipexole, but tricyclic antidepressants were found more efficacious than placebo.90,91 All 3 studies (large CI, intermediate CII, small CIII)92- 94 assessing pramipexole for depression in patients not requiring further treatment of motor symptoms were positive, but only the largest was placebo-controlled.
Although evidence is lacking for the use of specific SSRIs and SNRIs to treat patients with Parkinson disease–related depression, in our experience, both can be effective for this indication. Sertraline, escitalopram, citalopram, and extended-release venlafaxine are commonly used due to familiarity and low likelihood of major adverse effects. Although we find tricyclic antidepressants, in particular nortriptyline and desipramine, effective, they are used less frequently due to concerns about adverse effects in older patients who are cognitively impaired. Pramipexole is an option, especially when both motor and mood symptoms are being targeted.
Cognitive Impairment
Patients with Parkinson disease often develop mild cognitive impairment and may progress to Parkinson disease dementia (PDD). Few studies have assessed treatment of mild cognitive impairment in patients with Parkinson disease. A small CIV pilot study of atomoxetine in patients with Parkinson disease and executive dysfunction demonstrated a clinically significant improvement and a small CII study of atomoxetine for treatment of depression in patients with Parkinson disease reported improvements in global cognitive performance.95,96
Cholinergic dysfunction may be partially responsible for the cognitive impairment seen in the majority of patients with Parkinson disease over time.97 There are no published studies of cholinesterase inhibitors for Parkinson disease–related mild cognitive impairment, but a phase 4 RCT of the rivastigmine transdermal patch is currently under way (NCT01519271) and a study of donepezil for patients with Parkinson disease–related mild cognitive impairment and PDD is planned (NCT01014858). Rasagiline improved some measures of attention and verbal fluency in patients with Parkinson disease and mild cognitive impairment (1 intermediate CII study), suggesting a favorable benefit on executive function.98 A longer-term study is ongoing (NCT013823420).
A recent Cochrane review found that cholinesterase inhibitors are associated with improvements in global assessment, cognitive function, behavioral disturbance, and activities of daily living in patients with PDD.99 Five placebo-controlled trials have assessed cholinesterase inhibitors for PDD.70,100 Rivastigmine moderately improved measures of dementia, cognition, and behavioral symptoms (1 large CII trial). Three small studies of donepezil (2 CI and 1 CII) showed variable benefit, whereas 1 large CII study did not achieve its predefined primary end points, but secondary outcomes suggested that donepezil may improve cognition, executive function, and global status in PDD.100 There are no blinded RCTs of galantamine for PDD, but 1 small, open-label CIII study suggested benefit. Conflicting data exist for the use of memantine, a N-methyl-d-aspartate receptor antagonist, in PDD.70
In our experience, achieving clinically meaningful benefit with cholinesterase inhibitors in PDD is variable and unpredictable. Still, the response in some patients can be striking; therefore, a trial is warranted. However, exacerbation of tremor can limit their use. Gastrointestinal symptoms can also be problematic and the rivastigmine transdermal patch is generally better tolerated than the oral formulation. Response to memantine has been disappointing.
Orthostatic Hypotension
Orthostatic hypotension can be a major problem in Parkinson disease and should be regularly evaluated. Orthostatic hypotension can be an inherent component of Parkinson disease as a manifestation of autonomic dysfunction, but it can also be an adverse effect of dopaminergic medication. One small CII study reported improvement in symptoms of orthostatic hypotension and clinical global impression, focusing on orthostasis with use of fludrocortisone or domperidone compared with nonpharmacological measures; although not all participants had orthostatic hypotension at baseline.101
Midodrine was evaluated for treating neurogenic orthostatic hypotension in 3 studies (2 intermediate CI and 1 small CII) that included patients with Parkinson disease,102- 104 and a small CIII trial of pyridostigmine for orthostatic hypotension included 3 patients with Parkinson disease.105 Results were positive, but patients with Parkinson disease were not analyzed separately. A small crossover trial of yohimbine for orthostatic hypotension in patients with Parkinson disease was negative,106 whereas a small CII comparison study of yohimbine and pyridostigmine vs placebo involving some patients with Parkinson disease demonstrated a significant improvement only for yohimbine.107
In a small CIII study, indomethacin significantly reduced orthostatic symptoms and drop in blood pressure with standing.108 Droxidopa, a synthetic precursor of norepinephrine, caused an objective improvement in orthostatic hypotension but not of orthostatic hypotension symptoms in a placebo-controlled RCT in Parkinson disease and multiple system atrophy (published in abstract form only).109
In our experience, domperidone (not uniformly available in the United States) controls dopamine agonist–induced hypotension, especially when it occurs on the introduction of dopamine agonist treatment. It can also improve orthostatic hypotension worsened by levodopa. Before or in concert with trials of fludrocortisone and midodrine, we use nonpharmacological techniques for managing orthostatic hypotension, including increasing salt and fluid consumption, elevating the head of the bed, and compression stockings. Quickly drinking 2 8-ounce glasses of cold water can have a rapid ameliorative effect on orthostatic hypotension. Monitoring for supine hypertension is important when using antihypotensive drugs.
Sialorrhea
Atropine drops were effective for sialorrhea in 6 patients with Parkinson disease, but systemic adverse effects, including delirium and hallucinations, occurred (1 small CIII study).2 Ipratropium bromide spray did not improve objective or subjective measures of drooling following 2 weeks of treatment (1 small CI study),110 whereas glycopyrrolate significantly improved mean sialorrhea scores (1 small CI study).111 Although a pilot study (small CI) of intraoral tropicamide film112 failed to show benefit, a phase 2 study is currently under way (NCT01844648).
Treatment with botulinum toxin type A (BTA) injections significantly improved an objective measure of saliva quantity, measures of social embarrassment, and drooling frequency in a small CII study.113 Benefits were also reported in 2 small RCTs (1 CI and 1 CIII) of BTA for sialorrhea in mixed populations including patients with Parkinson disease.4,114 Botulinum toxin type B (BTB) injections reduced drooling, disability, and embarrassment related to sialorrhea (1 intermediate CI, 1 small CII, and 1 small CIV studies).115- 117 Injections of BTA and of BTB had similar effectiveness and safety in a small CII pilot study of patients with amyotrophic lateral sclerosis or Parkinson disease.118 Dysphagia is a potential adverse effect and may limit use.
In our experience, anticholinergic drugs with relatively low central activity may be useful but are poorly tolerated in older patients with cognitive dysfunction, whereas glycopyrrolate may be very effective and better tolerated due to limited blood-brain barrier penetration. Injections of BTA can be effective and are well tolerated; standard parotid injections can be supplemented with submandibular injections for improved efficacy but have potentially more complications.
DISCUSSION
In this review we have summarized the evidence from the literature addressing initial treatment and management of various disease- and treatment-related symptoms. Our recommendations are in agreement with several recently published guidelines on the management of Parkinson disease (eTable 3 in the Supplement). High-quality evidence is available for some treatment recommendations, for example initial therapy, but for other issues, such as management of dyskinesia, nausea, RBD, and orthostatic hypotension, data are limited. For these problems we have made recommendations based on our review of published evidence and our experience.
Levodopa is the most effective treatment for Parkinson disease. Figure 2, Figure 3, and Figure 4 provide a treatment algorithm for Parkinson disease motor symptoms based on age, symptom characteristics, and responses to treatment. Table 4 summarizes treatment options for motor symptoms including indications, level of recommendation for efficacy, doses, and adverse effects.
Many patients incorrectly believe that levodopa loses efficacy after 5 years or that it is toxic to dopamine neurons. These concerns, and a fear of developing motor complications, especially dyskinesia, often result in a “levodopa phobia.” However, dyskinesia is often mild and can usually be successfully treated.119 Age is especially important because younger patients develop dyskinesia earlier and more frequently following the initiation of levodopa, and older patients are more prone to cognitive and psychiatric adverse effects with all anti-Parkinson medication, requiring careful assessment of risk to benefit ratios. Dyskinesia and motor fluctuations that impair quality of life are indications for deep brain stimulation and this treatment should be considered in younger patients (generally <70 years) who retain a good response to individual doses of levodopa in the absence of cognitive impairment or active psychosis,120- 122 particularly before the development of intractable psychosocial impairment.
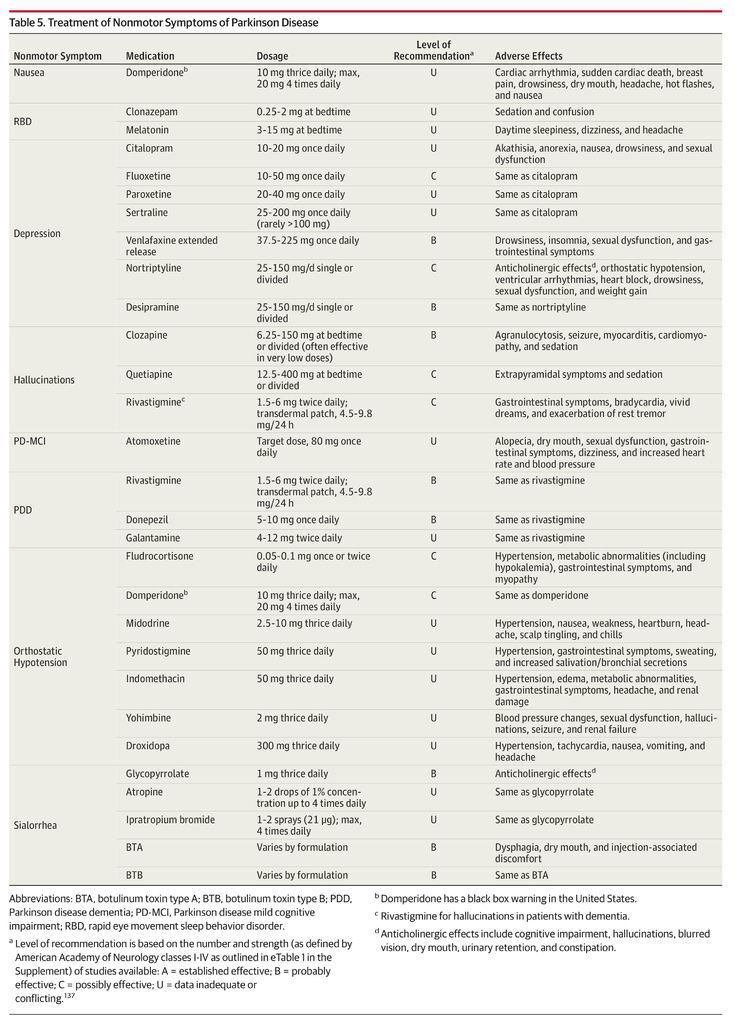
As Parkinson disease progresses, both nonmotor and motor symptoms emerge that are unresponsive to dopaminergic medication. Therapies are available for many nonmotor symptoms (Table 5), including cholinesterase inhibitors for PDD, antidepressants and pramipexole for depression, botulinum toxin injections for sialorrhea, and clozapine for hallucinations. However, axial motor symptoms, including falls, dysphagia, and postural instability tend to be treatment-resistant.
CONCLUSIONS
The quality of evidence for managing Parkinson disease motor and nonmotor symptoms and medication adverse effects is largely moderate. Study methods, inclusion and exclusion criteria, and outcome measures for most nonmotor features have been inconsistent and nonstandardized, and trial durations have been too short to establish extended efficacy and safety outcomes for these chronic problems. Further high-quality RCTs are needed. Finally, given its inexorable progression and complex, multifaceted disability, the greatest unmet therapeutic need is identification of effective neuroprotective and disease-modifying therapy. Multiple therapies are in development based on current hypotheses of disease pathogenesis.9
ARTICLE INFORMATION
Study concept and design: Connolly, Lang.
Acquisition, analysis, or interpretation of data: Connolly, Lang.
Drafting of the manuscript: Connolly.
Critical revision of the manuscript for important intellectual content: Connolly, Lang.
Administrative, technical, or material support: Connolly, Lang.
Study supervision: Lang.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Lang reports receiving financial compensation for consultancy work with Abbott, Abbvie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen-Idec, Boehringer-Ingelheim, Ceregene, Medtronic, Merck, NeuroPhage Pharmaceuticals, Novartis, and Teva; receiving grant funding from Brain Canada, the Canadian Institutes of Health Research, the Edmond J. Safra Philanthropic Foundation, the Michael J. Fox Foundation, the National Parkinson Foundation, Parkinson Society Canada, the Tourette Syndrome Association, and the W. Garfield Weston Foundation; and receiving royalties from Cambridge University Press, Johns Hopkins Press, Saunders, and Wiley-Blackwell. No other disclosures were reported.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 